Introduction
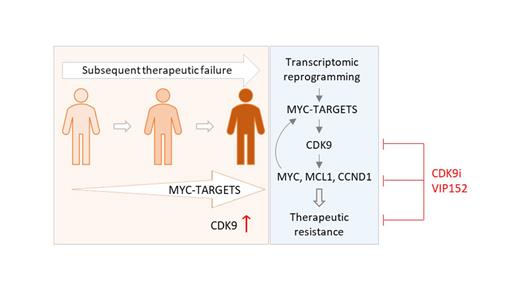
Mantle cell lymphoma (MCL) is an aggressive subtype of non-Hodgkin lymphoma. Even most advanced therapies are generally followed by resistance and relapse, creating an urgent need for novel drugs to overcome resistance. Our and other studies have shown transcriptome is abnormally reprogrammed in patients with therapeutic resistance. CDK9, as a master transcriptional regulator, is overexpressed in tumors after subsequent relapses to BTK inhibitors (BTKi) and CAR-T therapy. VIP152 is a highly potent and selective CDK9 inhibitor under preclinical and early clinical development. As yet, its potency and safety in patients with MCL, especially those with multiple therapeutic resistances, has not been addressed.
Method
We performed single-cell RNA sequencing and bulk RNA sequencing to investigate enriched cancer hallmarks in MCL. Cell viability assays were conducted in a time- and dose-dependent manner to assess the in vitro efficacy of VIP152. Apoptosis assays and western blots were used to identify relevant pathways in bypassing resistances. Cell line-derived xenograft and patient-derived xenograft models of MCL created in immunodeficient mice were used to monitor tumor growth and thus determine the in vivo efficacy of VIP152 in overcoming therapeutic resistances.
Results
Based on single-cell RNA sequencing and bulk RNA sequencing, we found that the cancer hallmark MYC-TARGETS is progressively enriched in MCL patients with subsequent failures to BTKi and CAR-T therapy. CDK9 was found to be overexpressed in CAR-T-relapsed patients compared to CAR-T-naïve but BTKi-refractory/relapsed patients. We hypothesized that CDK9 overexpression and activity may greatly contribute to the transcriptomic reprogramming in patients with therapeutic resistance. Targeting CDK9-mediated transcription with the specific CDK9 inhibitor VIP152 was highly effective in MCL cell lines at low nanomolar range (55-172 nM). VIP152 robustly induced apoptosis in a time- and dose-dependent manner, which was largely dependent on caspase-3 activity. It markedly inhibited CDK9 phosphorylation and down streaming signaling, and suppressed the expression of short-lived proteins like MYC, MCL-1, and cyclin D1 that are critical for MCL cell survival and proliferation. VIP152 effectively inhibited the tumor growth of MCL cell line-derived xenograft models in vivo (P<0.001) with no apparent adverse effects in mice. Moreover, VIP152 markedly inhibited the growth of three patient-derived xenograft tumor models, overcoming BTKi resistance (P<0.001), BTKi-venetoclax dual resistance (P<0.01), and BTKi-CAR-T dual resistance (P<0.001).
Conclusion
These data showed that CDK9 was a promising target for treating MCL and using VIP152 to specifically target it was efficacious in overcoming a variety of therapeutic resistances in MCL.
Disclosures
Wang:Bantam Pharmaceutical: Honoraria; VelosBio: Consultancy, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Pepromene Bio: Consultancy; Oncternal: Consultancy, Research Funding; Parexel: Consultancy; Milken Institute: Consultancy; Miltenyi Biomedicine: Consultancy; Merck: Consultancy, Honoraria; Eli Lilly and Company: Consultancy, Research Funding; Leukemia & Lymphoma Society: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; InnoCare: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; DTRM Biopharma (Cayman) Limited: Consultancy; Deciphera: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria; BioInvent: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Be Biopharma: Consultancy; AstraZeneca: Consultancy, Honoraria, Other: Travel, Research Funding; Amphista Therapeutics Limited: Consultancy; ADC Therapeutics America: Consultancy; Acerta Pharma: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; CAHON: Honoraria; Dava Oncology: Honoraria, Other: Travel; Eastern Virginia Medical School: Honoraria; Genmab: Honoraria, Research Funding; i3Health: Honoraria; IDEOlogy Health: Honoraria; Medscape: Honoraria; MD Education: Honoraria; Meeting Minds Experts: Honoraria; MJH Life Sciences: Honoraria; Moffit Cancer Center: Honoraria; NIH: Honoraria; Nurix: Honoraria; Oncology Specialty Group: Honoraria; OncLive: Honoraria; Physicians Education Resources (PER): Honoraria, Other: Travel; Practice Point Communications (PPC): Honoraria; Scripps: Honoraria; Studio ER Congressi: Honoraria; WebMD: Honoraria; Celgene: Other: Travel, Research Funding; Genentech: Consultancy, Research Funding; Juno Therapeutics: Research Funding; Loxo Oncology: Consultancy, Research Funding; Molecular Templates: Research Funding; Vincerx: Research Funding; Anticancer Association: Honoraria; BGICS: Honoraria; Clinical Care Options: Honoraria; Epizyme: Consultancy, Honoraria; Hebei Cancer Prevention Federation: Honoraria; Imedex: Honoraria; TS Oncology: Honoraria; Mumbai Hematology Group: Honoraria; OMI: Honoraria; Pharmacyclics: Honoraria; Physicians Education Resources: Honoraria; Practice Point Communications: Honoraria; CSTone: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal